- in popular sizes they are relatively inexpensive and easy to find
- starting the assembly is easy as the threads are standard until the nylon ring is encountered
- prevailing torque is consistent and usually low within the tolerance band
- there is no risk of galling or seizure during assembly
The locking element is usually made of Nylon, specifically 66 Nylon or Zytel® 101. The rings are normally cut from sheets using dies, which makes their dimensions very consistent. The dimensional consistency is reflected in prevailing torque consistency.
The prevailing torque is developed when the male (bolt) thread interferes with the nylon ring and forms the complimentary female thread into the ring with a combination of elastic and plastic deformation. The outside diameter of the ring is constrained, so the inside diameter must allow this deformation without overfilling the mating thread, or the ring can be pushed out of the nut. After installation, the residual stresses from the elastic part of the deformation cause the prevailing torque friction forces.
So, all this is well understood, and well controlled, and Nylon insert locknuts are the perfect locknuts, right? Not always. They may not be the best option if the assembly or storage or service environment of the application includes some combination of
- elevated temperature
- elevated relative humidity
- exposure to ultraviolet radiation, including sunlight
- extended time
- exposure to some chemicals
The following are excerpted from DuPont™ Minlon® and Zytel® nylon resins Design Information – Module II. I recommend browsing this guide and evaluating any application with respect to its recommendations before selecting a nylon insert locknut for the application.
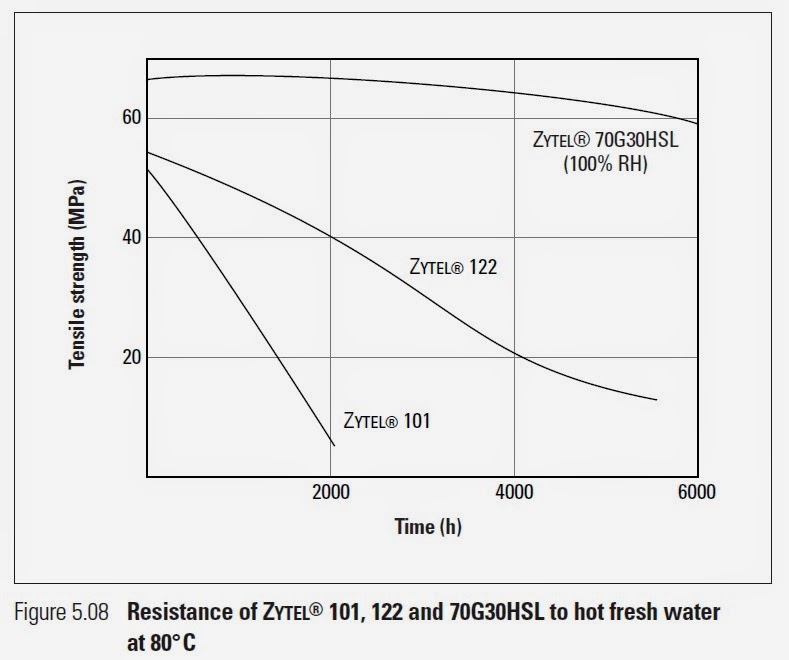
"Nylons absorb moisture from the air and 66 nylon equilibrates at about 2,8% water at 50% RH and at about 8,5% at 100% RH. This plasticizes the nylon, lowering its strength and stiffness but increasing its toughness and elongation. Moisture absorption increases dimensions of 66 nylons by 0,6% at 50% RH and about 2,6% at 100% RH. The process is reversible, that is, the strength and stiffness increase and dimensions decrease as moisture content decreases. Absorption and desorption are slow processes. For example, it takes about 125 days for a 1,5 mm thick dry specimen to reach equilibrium moisture content when exposed to 50% relative humidity."
"Properties observed in weathering studies
Moulded test parts exposed outdoors to ultraviolet radiation may ultimately fail for one of the following reasons: (1) loss of strength, (2) loss of toughness or (3) change in appearance.
Changes in tensile and yield strength over the time period studied were determined using ASTM D638. Toughness was measured using a mandrel bend test, in which test bars are bent rapidly 180° around a 3,2 mm diameter steel mandrel. A tough bar has the capability of being deformed in this manner without breaking.
The relative viscosity of nylon is related to its molecular weight. Exposure of nylon that is inadequately stabilized against ultraviolet light results in surface degradation with a corresponding drop in relative viscosity or molecular weight. The interest in relative viscosity accrues from the fact that serious loss in this property is related to a comparable loss in toughness.
Change in appearance has been measured by colour difference using Adams units which are similar to National Bureau of Standards (NBS) units."
"Factors important to service life of a nylon in a chemical environment. The designer must define the specific conditions of the chemical environment before he can determine whether the probability for a successful application is good. Some of these conditions are:
- Temperature. Depending on the specific reagent, service life can be significantly reduced by an increase in temperature. Acids and oxidizing agents are particularly harmful to nylons at higher temperatures. It is difficult to generalize on the quantitative aspects of increased temperatures although a 15° C rise in temperature will frequently reduce service life by 25–50%.
- Chemical concentration. Chemical concentration has a bearing on service life of nylons. This is true for acids and will depend greatly upon the pH. The effect of concentration will vary from one material to another and generalizations are impossible to make.
- Time. This is important in defining the suitability of an application in a particular reagent. Does the application involve 60 days of intermittent exposure or two years of continuous exposure?
- Part surface to weight ratio. Here the ratio of surface area to weight is important. The greater this ratio, the more rapid the attack.
- Stress level. Although nylons are very resistant to attack from a wide variety of chemical agents, a few inorganic salts can cause severe breakdown of nylons under stress.
Zinc chloride, for example, is especially harmful to 66 nylons such as ZYTEL® 101, but has a lesser effect on 612 nylons such as ZYTEL® 151. End-use tests should always be employed to determine the suitability of a nylon for a particular application."




No comments:
Post a Comment